Cl- Ion Number Of Electrons . Web we know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Web in this video we’ll use the periodic table and a few simple rules to find the number of. Web the chloride ion, cl−, has a charge of −1, meaning, it had gained 1 electron in its outermost orbital. Web some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Determine the number of protons and electrons in an. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine. Web describe the locations, charges, and masses of the three main subatomic particles. Web the electron configuration of a chlorine atom (#cl#) is as follows: Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any.
from general.chemistrysteps.com
Web we know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Web the electron configuration of a chlorine atom (#cl#) is as follows: Web describe the locations, charges, and masses of the three main subatomic particles. Web the chloride ion, cl−, has a charge of −1, meaning, it had gained 1 electron in its outermost orbital. Web in this video we’ll use the periodic table and a few simple rules to find the number of. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine. Web some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any. Determine the number of protons and electrons in an.
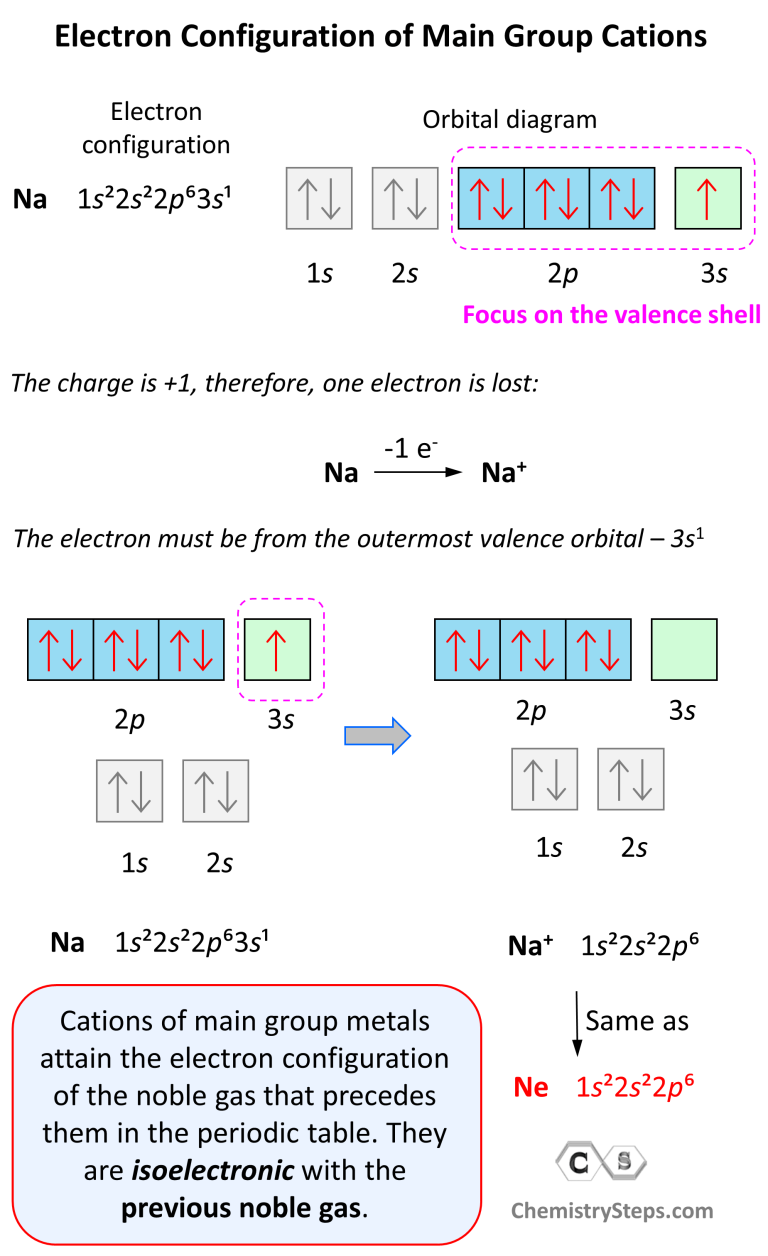
Electron Configurations of Ions Chemistry Steps
Cl- Ion Number Of Electrons Web describe the locations, charges, and masses of the three main subatomic particles. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any. Web the electron configuration of a chlorine atom (#cl#) is as follows: Determine the number of protons and electrons in an. Web we know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Web describe the locations, charges, and masses of the three main subatomic particles. Web some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web the chloride ion, cl−, has a charge of −1, meaning, it had gained 1 electron in its outermost orbital. Web in this video we’ll use the periodic table and a few simple rules to find the number of. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Cl- Ion Number Of Electrons Web in this video we’ll use the periodic table and a few simple rules to find the number of. Web some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web describe the locations, charges, and masses of the three main subatomic particles. Web the chloride ion, cl−, has. Cl- Ion Number Of Electrons.
From www.wikihow.com
How to Find Electrons 7 Steps (with Pictures) wikiHow Cl- Ion Number Of Electrons Web some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any. Determine the number of protons and electrons in an. Web in this. Cl- Ion Number Of Electrons.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Cl- Ion Number Of Electrons Web in this video we’ll use the periodic table and a few simple rules to find the number of. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine. Determine the number of protons and electrons in an. Web the chloride ion, cl−, has a charge of −1, meaning, it had gained 1 electron in its outermost orbital. Web the electron configuration. Cl- Ion Number Of Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Cl- Ion Number Of Electrons Web in this video we’ll use the periodic table and a few simple rules to find the number of. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any. Determine the number of protons and electrons in an. Web describe the locations, charges, and masses of the three main subatomic particles. Web. Cl- Ion Number Of Electrons.
From circuitdiagramhits.z21.web.core.windows.net
Orbital Diagram For Cl Cl- Ion Number Of Electrons Web some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web we know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Web the chloride ion, cl−, has a charge of −1, meaning, it. Cl- Ion Number Of Electrons.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Cl- Ion Number Of Electrons Web some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web the chloride ion, cl−, has a charge of −1, meaning, it had gained 1 electron in its outermost orbital. Web in this video we’ll use the periodic table and a few simple rules to find the number. Cl- Ion Number Of Electrons.
From www.coursehero.com
Lewis Symbols and Structures Chemistry I Course Hero Cl- Ion Number Of Electrons #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine. Determine the number of protons and electrons in an. Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any. Web the electron configuration of a chlorine atom (#cl#) is as follows: Web the chloride ion, cl−, has a charge of −1, meaning, it had. Cl- Ion Number Of Electrons.
From byjus.com
How to find the number of electrons/ions Cl- Ion Number Of Electrons Web in this video we’ll use the periodic table and a few simple rules to find the number of. Web the chloride ion, cl−, has a charge of −1, meaning, it had gained 1 electron in its outermost orbital. Web some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an. Cl- Ion Number Of Electrons.
From socratic.org
How does ionic radius change across a period? Socratic Cl- Ion Number Of Electrons Web the electron configuration of a chlorine atom (#cl#) is as follows: #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine. Determine the number of protons and electrons in an. Web in this video we’ll use the periodic table and a few simple rules to find the number of. Web the chloride ion, cl−, has a charge of −1, meaning, it. Cl- Ion Number Of Electrons.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Cl- Ion Number Of Electrons Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any. Web in this video we’ll use the periodic table and a few simple rules to find the number of. Web describe the locations, charges, and masses of the three main subatomic particles. Web the electron configuration of a chlorine atom (#cl#) is. Cl- Ion Number Of Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Tellurium (Te2, Te4+) Cl- Ion Number Of Electrons Web some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web we know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Web this electron configuration calculator will instantly show you the distribution of. Cl- Ion Number Of Electrons.
From studygripewater.z21.web.core.windows.net
How To Find Valence Number Cl- Ion Number Of Electrons Determine the number of protons and electrons in an. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine. Web the electron configuration of a chlorine atom (#cl#) is as follows: Web in this video we’ll use the periodic table and a few simple rules to find the number of. Web describe the locations, charges, and masses of the three main subatomic. Cl- Ion Number Of Electrons.
From wisc.pb.unizin.org
6.4 Electronic Structure of Atoms (Electron Configurations) Chemistry Cl- Ion Number Of Electrons Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any. Web we know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Web some atoms have nearly eight electrons in their valence shell and can gain additional valence. Cl- Ion Number Of Electrons.
From ceusyfvy.blob.core.windows.net
Chlorine Has Isotopes Cl35 And Cl37 at Wayne Otto blog Cl- Ion Number Of Electrons Web the chloride ion, cl−, has a charge of −1, meaning, it had gained 1 electron in its outermost orbital. Web the electron configuration of a chlorine atom (#cl#) is as follows: Determine the number of protons and electrons in an. Web some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. Cl- Ion Number Of Electrons.
From wiredatapoliciaj0.z22.web.core.windows.net
Orbital Diagram Of Cl Cl- Ion Number Of Electrons #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine. Web we know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Web this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any. Determine the number of protons and electrons in. Cl- Ion Number Of Electrons.
From wiredatagotykw0.z21.web.core.windows.net
Atomic Diagram Of Chlorine Cl- Ion Number Of Electrons Web describe the locations, charges, and masses of the three main subatomic particles. #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine. Web the chloride ion, cl−, has a charge of −1, meaning, it had gained 1 electron in its outermost orbital. Web some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they. Cl- Ion Number Of Electrons.
From fwrnaissnschematic.z22.web.core.windows.net
Atomic Diagram Of Chlorine Cl- Ion Number Of Electrons Web we know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Determine the number of protons and electrons in an. Web the chloride ion, cl−, has a charge of −1, meaning, it had gained 1 electron in its outermost orbital. Web the electron configuration of. Cl- Ion Number Of Electrons.
From masterconceptsinchemistry.com
How to calculate the number of electrons in an ion Cl- Ion Number Of Electrons #1s^2 2s^2 2p^6 3s^2 3p^5# but a chlorine. Web some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Web we know that the mass number (a) = number of protons + the number of neutrons, and therefore, the number of protons is equal to: Web in this video. Cl- Ion Number Of Electrons.